Alkanes
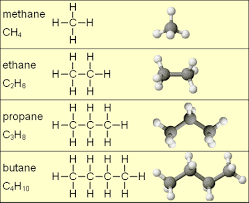
Alkanes Alkanes are saturated organic compounds with G.M.F. (General Molecular Formulae) CnH2n+2, where; n is the number is the number of carbon atom and is greater or equal to 1 C is the carbon atom or symbol H is the hydrogen atom or symbol All compounds in this serie have the same chemical properties because they all exhibit the same molecular formulae. Methane is the first member in this serie and other members includes; Ethane, Propane, Butane, Pentane, Hexane, Heptane, Octane, Nonane, decane... I recommend the format below for ease of remembrance. M- for Methane E- for Ethane P- for Propane B- for Butane the second P- stands for Pentane H- for Hexane the second H- stands for Heptane O- for Octane N- for Nonane D- for Decane So, at the end we should have MEPBPHHOND respectively. Note that; the first four members of the alkanes are gases while other memebers are liquids. Now, let us discuss briefly about some of the members in the alkane serie. 1. METHANE- CH4...