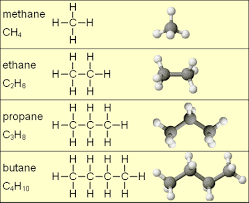
Alkanes
Alkanes are saturated organic compounds with G.M.F. (General Molecular Formulae) CnH2n+2, where; n is the number is the number of carbon atom and is greater or equal to 1
C is the carbon atom or symbol
H is the hydrogen atom or symbol
All compounds in this serie have the same chemical properties because they all exhibit the same molecular formulae. Methane is the first member in this serie and other members includes; Ethane, Propane, Butane, Pentane, Hexane, Heptane, Octane, Nonane, decane... I recommend the format below for ease of remembrance.
M- for Methane
E- for Ethane
P- for Propane
B- for Butane
the second P- stands for Pentane
H- for Hexane
the second H- stands for Heptane
O- for Octane
N- for Nonane
D- for Decane
1. METHANE- CH4
Methane has a general molecular formulae of C1H2(1)+2, using the GMF of the alkane(CnH2n+2). It is the major constituent of natural gas which is normally found above petroleum during fuel exploration. Methane is the major gas in some planets like Jupiter, Saturn, Uranus, Neptune and most especially Pluto which consists mainly of methane gas.
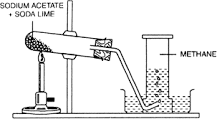
Preparation
Methane is prepared in the laboratory by heating anhydrous sodium ethanoate(ester) with an alkali such as soda lime(NaOH).
Soda-lime is prepared by slaking quicklime with a solution of concentrated sodium hydroxide. It is used in preference to caustic soda due to its nature (it is not deliquescent and does not attack glass so readily.)
Anhydrous sodium ethanoate also known as sodium acetate is ground with an equal mass of soda-lime and then heated in a test tube. Methane gas is usually given-off during the reaction and is collected over water.
Physical Properties of Methane
1. Methane is neutral to litmus paper
2. It is less dense than air
3. It is slightly soluble in water
4. It is a colorless & odorless gas
Chemical Properties of Methane
1. It burns readily in air to produce Carbon IV oxide and steam.
CH4 + 2O2 -----> CO2 + 2H2O
Note: the general equation for combustion is given as; CxHy + (x+y/4)O2 ----> xCO2 + 2H2O
2. Substitution reaction- in such a reaction, there is a direct displacement of an atom or group of atom by another atom or group. Methane and other member of alkane under-go substitution reaction with halogens to produce 'halo-alkanes'. For example, methane under-go chlorination in a four-step reaction to yield tetrachloromethane as the final product. This reaction is catalysed by ultraviolet rays or sunlight therefore, it is a photolytic reaction. Here is how the reaction goes
CH4 + Cl2 ------> CH3Cl + HCl
CH3Cl + Cl2 ------> CH2Cl2 + HCl
CH2Cl2 + Cl2 ------> CHCl3 + HCl
CHCl3 + Cl2 ------> CCl4 + HCl
The reaction continues in the presence of U.V. rays until CCl4 is produced.
Uses
1. Methane is used as the starting material for the production of many important organic compounds such as ethanol, ethanoic acid, methoxyl methane (ether).
2. Trichloromethane is a product of chlorination of methane and it is used as an anaesthetic during surgical operations.
3. Tetrachloromethane is also another product of the chlorination of methane and is used as an organic solvent.
4. It is the major constituent of natural gas used as domestic fuel for cooking.
Ethane- C2H6
Ethane is the second member in the Alkane series, with a G.M.F. of C2H2(2)+2 = C2H6. Ethane is the most used gas in the alkane series as it has diverse of uses in the world of chemistry. While working with electrolysis, famed English scientist Michael Faraday mistook the hydrocarbon compound for methane back in 1834. Another error, just a decade later, resulted in a separate set of scientists once again misidentifying the compound as methyl. It was later in 1864 that the ethane gas was correctly identified.
Laboratory and Industrial Preparation of Ethane
• Crude Oil and natural gas (consisting mainly of and other impurities from the gas stream. methane) are mostly found together and is normally processed to remove various impurities. Natural gas processing removes hydrocarbons like ethane, butane, propane and other hydrocarbons. Once separated, the valuable hydrocarbons are liquefied under pressure and stored in container where they can be sold as bottled gases for various purposes including fuel and feedstock. Note that ethane and other hydrocarbons are not always separated during refining of natural gas, as it is a costly process to separate the hydrocarbons from methane (natural gas). This is the industrial preparation of the ethane gas as this cannot be done in small laboratories.• Ethane gas can also be obtained in the laboratory when an addition reaction occurs between ethene and hydrogen gas.
C2H4(g) + H2(g) -------- C2H6(g)
The opportunities
Today, ethane is a feedstock in many petrochemical processes, and is considered a valuable commodity. Regardless, energy economics ultimately drive whether ethane is removed and sold or whether it remains in the gas stream and is burned by the end user. If ethane is not removed, it becomes a wasted resource, which is not in the interest of energy producers and manufacturers.Recent advances in drilling techniques have resulted in increased production of energy resources throughout the U.S. As a result, prolific supplies of the commodity are rejuvenating American manufacturing and creating the first opportunity to export ethane globally.
Benefits of ethane to life
Ethane is a clean burning fuel and can be liquefied at a higher pressure, which decreases infrastructure transport costs. As a result, ethane is being looked to as a more efficient fuel source for fast growing and developing countries.In some markets with lack of infrastructure or limitation of capacity, Ethane is more affordable than LNG for power generation due to cheaper liquefaction, transportation and regasification costs.
LEG is more feasible than LNG for power generation due to its price formula structure, unlinked from crude oil prices.
American Ethane Company provides long-term supply from reliable sources via controlled supply chain, so the customer is assured of the ethane supply to its power plant.
Also, the ethane gas are very useful fuel which can be used for powering engines such as aircraft engines and others.



Comments
Post a Comment