Organic Chemistry
Introduction to Organic Chemistry
Organic Chemistry is a branch of chemistry which deals basically with the scientific study of organic compounds (i.e. compounds of carbon, hydrogen, and few other elements such as chlorine, fluorine, Iodine, nitrogen, sulphur, phosphorus etc.), their structures, properties and chemical reactions.• Study of structure is used to determine their chemical composition and formulae.
Structure according to the Oxford Advanced Learner’s Dictionary “is a thing that is made of several parts connected together, arranged, or organized”. Hence, studying the structure of organic compounds is the determining of its chemical composition.
• Study of properties- physical and chemical properties
Physical properties is based on the structural formulae, while the chemical properties is based on the molecular formulae. It should be noted that; Organic compounds which have the same molecular formulae usually have the same chemical properties while those having the same structural formulae share the same physical properties.
• Study of Organic reactions-
includes the chemical synthesis of natural products, drugs, and polymers as well as the theoretical study of organic molecules in the laboratory.
Probing further to organic chemistry, we can say, it is the study of compounds obtainable from plants and animals. In recent times, organic chemistry has had a great impact to the betterment and development in the World’s production and day-to-day activities. It is through the knowledge of organic chemistry and hydrocarbons we are able to explore Petroleum which has been the major source of energy after the Sun, this has immensely contributed to growth and development of nations and her citizens- it application includes; the production of wonder drugs, textiles, plastics, food and its preservation, automobile, fuel, domestic cooking gas, and even ammunition. Nevertheless, the ultimate source of organic compounds is the SUN. In the presence of ultraviolet rays, the enzymes in plants becomes activated which then aids the occurrence of photosynthesis thereby producing certain hydrocarbon which can later be acted upon by heat and pressure to produce complex organic compounds.
As discussed earlier, there are lot of variety of organic compounds, some of which have members of the halogen group (fluorine, chlorine, bromine, iodine, astatine) attached to their carbon atom, some which have no other element than hydrogen and carbon and others which have other elements like Sulphur, Nitrogen, Oxygen, Phosphorus, and so on.
In my previous article, I discussed about hydrocarbons and it was defined as any compound what so ever consisting mainly of hydrogen and carbon. You should note that; all hydrocarbons are organic compounds, but not all organic compounds are hydrocarbons, due to the presence of other elements.
Characteristics Feature of Carbon and Its Effect to Organic Chemistry
1. Carbon has the electronic configuration 1S2, 2s2, 2p2 which otherwise can be written as 2,4 thereby having 4 valence electrons and belonging to group 4, period 2 in the periodic table. If this is not clear to you, please read How to determine the group and period of elements from their electronic configuration. This nature gives it the ability to form covalent compounds with elements like hydrogen.2. The bonds which can exist between carbon and other compounds can either be single as in alkanes, double as in alkenes and triple as in alkynes. This has to do with the hybridization of organic compounds.
3. The ability of carbon atoms to form long branched chains i.e. to catenate is also another exceptional character of carbon. This as well has to do with octane number of fuels.
4. Slightly high energy is needed to break carbon-to-carbon bonds.
5. The intermolecular force between carbon-to-carbon atom is weak, hence little amount of energy is needed to break or weaken it.
6. Carbon does not have the ability to conduct or transmit electricity since it is a non-metal.
General Characteristics of Organic Compounds
1. All organic compounds have lower boiling and melting point compared to inorganic compounds and this is due to the weak intermolecular forces which exist between them.
2. Most organic compounds are volatile (i.e. they evaporate easily on exposure to air)
3. Generally, the solvent of most organic compounds are organic compounds as well. For example, artificial rubber (neoprene) and even natural rubber (isoprene) used in the making of car tyre can be dissolved by benzene which is an organic compound.
4. Only organic compounds which exhibits the (-OH) hydroxyl group and strongly electronegative elements like the halogens are readily soluble in water while other organic compounds are non-polar and are not soluble in water... See why OH group are soluble in water
5. Finally in this section, the reactivity of organic compounds are mostly slow and require external forces like catalyst, pressure, heating to speed up the reaction rate.
Now, let’s have a review of what we will be discussing in the remaining part of this article.
OCTANE NUMBER
The term ‘Octane number’ basically means the tendency of fuel to cause knocking to engines. A poor quality is usually more volatile than a quality fuel, it tends to burn faster in combustion engines. For fuel to be quality, it should have complex branched chain carbon atom. The best example of this is 2,2,4-trimethylpentane (iso-octane) an isomer of octane. Hence, the name “octane number”. High quality fuel have more weight compared to poor quality fuel. Increasing the octane number of fuel can easily be done by adding petrol additives e.g. methanol or ethanol, tetraethyl lead IV Pb(C2H5). Pb(C2H5) is a major pollutant to the environment. Moreover, there are many other quality petroleum additive available in the market.Homologous Series
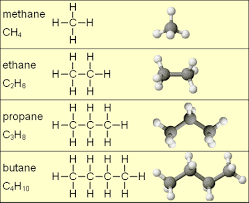
The homologous series in organic chemistry is the grouping of organic compounds which exhibits same molecular formulae. Homologous series includes:1. Alkane
2. Alkene
3. Alkyne
4. Alkanol
5. Alkanoic
6. Alkanone
7. Alkanoate
8. Alkanal
9. Amine
10. Amide
I’ll include the following to my list, since their individual compounds have the same General Molecular Formulae (G.M.F.)
11. Carbamide (Urea)
12. Protein
13. Carbohydrate
Functional Group
Functional group are usually attached to organic compounds. Functional groups includes:1. Hydroxyl group -OH
2. Alkyl group also known as Ligand -CH2
3. Carboxyl group -COOH
4. Carbonyl group -CHO
5. Amino group -NH2
For example, the Amines have the functional group -NH2 attached to its carbon atom, the alkanoic has the carboxyl group –COOH, alkanone has the carbonyl group –CHO, the alkanol has the hydroxyl group –OH.
Saturated & Unsaturated Organic Compounds
Organic compounds are said to be saturated if the compound’s carbon chain is completely filled up and exhibiting single bonds while all unsaturated organic compounds are those whose carbon atom has about two or three bonds i.e. double or triple bonds respectively. The saturated organic compounds includes the alkanes since they have single bond and are completely filled up.Testing for Saturation & Unsaturation
1. Unsaturated organic compounds decolorize bromine water- HOBr. The reddish-brown color of HOBr is always decolorized whenever it reacts with unsaturated compounds like Alkenes, Alkenes, or Alkanal. Meanwhile, saturated compounds do not affect the color of HOBr.2. Generally, all unsaturated compounds changes or bleaches the purple color of potassium permanganate (KMnO4) to green, the orange color of potassium heptaoxochromate (K2Cr2O7) and the color of Fehling solution.
With this, you should have a solid foundation in Organic chemistry. Wish you success and good luck!
Kindly comment your questions, modification or contributions while you share this educative article to friends and family. Also, follow this site by Email to receive the latest content published on this site delivered directly into your mailbox.



Comments
Post a Comment